6. Requesting new terms¶

6.1. Main Objective¶
Terms could be missing from public ontologies of choice. Where needed, new terms can be requested for these ontologies. The objective of this recipe is to provide a general guidline on how to request new terms, as well as to give some examples for specific ontologies.
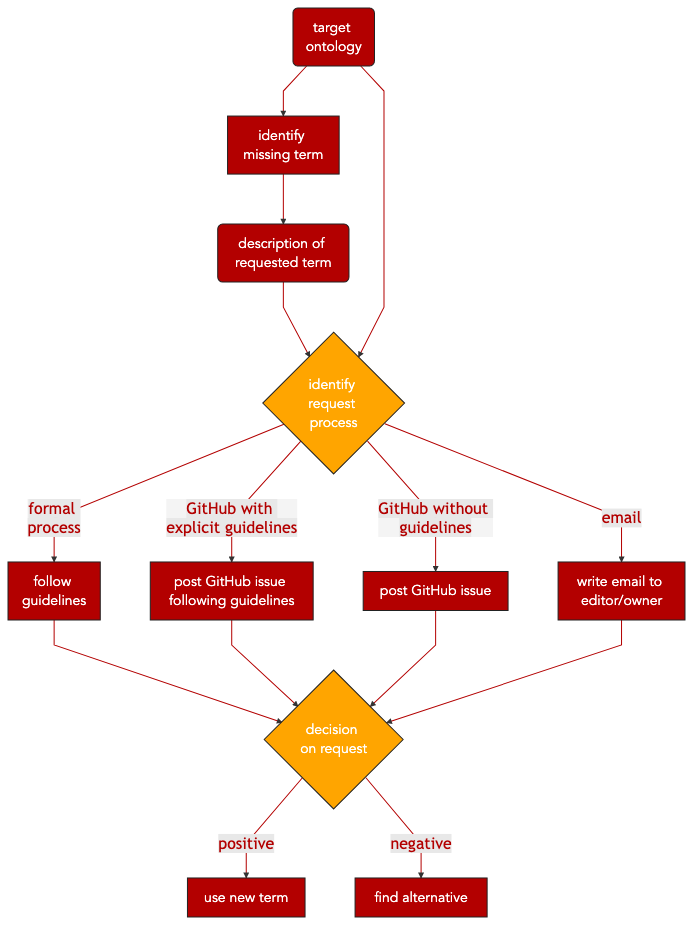
Requesting new terms in public (biomedical) ontologies might be a structured, streamlined process or completely undocumented, depending on the actual ontology. Also, the process can take somewhere between days and up to a year, depending on the implemented release cycles of the target ontology.
Some ontologies, often large projects organized by consortia, have a detailed formal request process, maybe even with a dedicated ticketing and tracking system. A big number of ontologies rely on GitHub as a publicly accessible ticketing system for reporting issues on the ontology and also for requesting new terms. They might provide explicit guidelines on how to create an issue for a new term request, or they rely on GitHub issues without guidelines. And there are also ontologies relying on email for requesting new terms. These different request processes can be summarized as follows:
Formal request process
Request via GitHub with explicit guidelines
Request via GitHub without guidelines
Request via email
In most ontologies, everybody can request new terms. However, in some ontologies only members have access to the request process. In case of a request process organized via GitHub, a free GitHub account is required.
6.2. Ingredients¶
List of new terms
Target ontology
E.g. SNOMED CT, MedDRA, OBI, EFO, BAO, CL, RxNorm, BTO - BRENDA, DCAT 2
6.4. FAIRification Objectives, Inputs and Outputs¶
Actions.Objectives.Tasks |
Input |
Output |
|---|---|---|
new term(s), ontology |
6.5. Table of Data Standards¶
Data Formats |
Ontologies |
|---|---|
Release Format 2 |
|
Rich Release Format |
6.6. Step-by-Step process¶
Step 1 Identify and describe relevant terms not included in public ontology of choice.
Step 2 Identify request process of the public ontology (i.e. formal request process, GitHub request with guidelines, GitHub request without guidelines, email request).
Step 3 Prepare at least the following information for requesting a new term:
justification for the request
preferred term label: a unique, unambiguous label for the term
potential alternative terms: common synonyms or translations
textual definition: expresses the meaning of the term, add sources and fully expand abbreviations
logical definition: suggest parent and child terms
example of usage: propose a use case
attribution: contributor names (and ORCIDs)
Step 4 Finalize and submit term request. Depending on the ontology, it can take up to a year to have the new term incorporated in the ontology, or to have the request rejected.
6.7. Examples¶
6.7.1. Formal request process¶
Example ontology: SNOMED CT - Systematized Nomenclature of Medicine
Scope of the ontology: conditions, clinical findings, procedures, body structures, substances, pharmaceuticals, devices, specimen
Information about requesting changes or additions: SNOMED CT has a Content Request Service (CRS). Requests can be submitted by members of SNOMED International, Nationale Release Centers or other authorized users, and must align with the Editorial guide. When submitting a request, it is important to follow the aspects mentioned in Step 3 of the Step-by-Step process. In addition to these aspects, the following is important to provide as well:
Reference(s) from a scientific or professional journal, or professional society
Fully expanded abbreviations
When submitting to the CRS, a request can have one of the sixteen possible statuses (‘New’, ‘Draft’, ‘Accepted’, ‘Under Authoring’, ‘Ready for Release’, ‘In Inception’, ‘Clarification Needed’, ‘Pending Internal Input’, ‘On Hold’, ‘Forwarded’, ‘Withdrawn’, ‘Rejected’, ‘Completed’, ‘Appeal’,’Appeal rejected’, ‘In Appeal Clarification’). Within CRS, submitters are notified when a status has been changed.
6.7.2. Request via GitHub with explicit guidelines¶
Example ontology: OBI - Ontology for Biomedical Investigations
Scope of the ontology: assays, devices, objectives in scientific investigations
Information about requesting changes or additions: OBI provides a GitHub repository and a mailing list. New-term requests are handled as GitHub issues. There is an explicit guideline document on how to request new terms.
For a proposed new term, they ask for the following information:
editor preferred term: a unique, unambiguous label for the term in American English
alternative terms: common synonyms or translations
textual definition
definition source for the textual definition
logical definition (or parent term)
example of usage
term editor: your name, and that of any collaborators, as it should appear in OBI
6.7.3. Request via GitHub without guidelines¶
Example ontology: CL - Cell Ontology
Scope of the ontology: cell types
Information about requesting changes or additions: The Cell Ontology provides a GitHub repository, a contact email, and a mail list. New-term requests (NTR) are formulated as issues on the GitHub repository.
6.7.4. Request via email¶
Example ontology: RxNorm
Scope of the ontology: drugs
Information about requesting changes or additions: Information about requesting terms can be found in the FAQ section of the Unified Medical Language System (UMLS) where RxNorm is part of.
Changes or additions to UMLS can be requested by contacting NLM Customer Support. The NLM Customer Support can be contacted through a form. If additions are specific to the source, you should contact the terminology source provider. Contact information is available in Appendix 1 of the Licence agreement.
UMLS is updated in May and November of each year.
6.8. References¶
References
6.8.1. What to read next?¶

FAIRsharing records appearing in this recipe:
- BRENDA Enzyme Database (BRENDA)
- BRENDA tissue / enzyme source (BTO)
- BioAssay Ontology (BAO)
- Cell Ontology (CL)
- Data Catalog Vocabulary (DCAT)
- Experimental Factor Ontology (EFO)
- GitHub
- Medical Dictionary for Regulatory Activities Terminology (MedDRA)
- OBO Foundry (OBO)
- Ontology for Biomedical Investigations (OBI)
- RxNORM
- Systematized Nomenclature of Medicine-Clinical Terms (SNOMEDCT)
- Web Ontology Language (OWL)
6.9. Authors¶
Authors
Name |
ORCID |
Affiliation |
Type |
ELIXIR Node |
Contribution |
|---|---|---|---|---|---|
CEMM |
Writing - Original Draft |
||||
Emma Vos |
The Hyve |
Writing - Original Draft |
|||
University of Oxford |
Writing - Review & Editing, Conceptualization |